Source: Years after Harvard scandal, U.S. pours millions into tainted field
A Reuters Investigation
Years after Brigham-Harvard scandal, U.S. pours millions into tainted stem-cell field
Medical scientist Piero Anversa gained global attention for his claim in 2001 that adult stem cells can regenerate hearts. That year, he spoke in a TV interview with journalist Charlie Rose.
Faked heart studies by a once-obscure scientist duped the U.S. government and medical establishment for years. Washington is still paying for it.
Mario Ricciardi, a young Italian molecular biologist, was thrilled when he was selected to work with one of Harvard Medical School’s most successful stem cell researchers.
His new boss, Dr. Piero Anversa, had become famous within the field for his bold findings in 2001 that adult stem cells had special abilities to regenerate hearts or even cure heart disease, the leading cause of U.S. deaths . Millions in U.S. government grants poured into Anversa’s lab at Brigham and Women’s Hospital in Boston. Top journals published his papers. And the American Heart Association (AHA) proclaimed him a “research pioneer.”
“He was like a god,” recalled Ricciardi, now 39, one of several scientists to speak out for the first time about their experiences in Anversa’s lab.
Within a year of Ricciardi’s arrival in 2011, they grew suspicious, the scientists recalled. They couldn’t replicate the seminal findings of their celebrated boss and became concerned that data and images of cells were being manipulated. Anversa and his deputy gruffly dismissed their questions, they said.
They took their concerns to Brigham officials, telling them that Anversa’s blockbuster results appeared to have been faked. “The science just wasn’t there,” Ricciardi said.
After an investigation lasting almost six years, Brigham and Harvard wrote in a two-paragraph statement that they had found “falsified and/or fabricated data” in 31 papers authored by Anversa and his collaborators. In April 2017, the U.S. Justice Department separately concluded in a civil settlement with Brigham that Anversa’s lab relied on “the fabrication of data and images” in seeking government grants and engaged in “reckless or deliberately misleading record-keeping.”
Yet federal money has continued to flow to test the proposition advanced by Anversa – that adult stem cells can regenerate or heal hearts. Over two decades, federal and private grants have streamed into research labs despite allegations of fraud and fabrication against Anversa and others in the field, Reuters found. Meanwhile, no scientist has credibly established that Anversa’s regeneration hypothesis holds true in humans, according to researchers and a review of medical literature.
Since 2001, the U.S. National Institutes of Health spent at least $588 million on such heart research, Reuters found in an analysis of government data. More than $249 million, about 43% of the total, has been awarded since March 2013. By that time, the federal government had been informed of the fabrication allegations against Anversa, according to documents and interviews with sources familiar with the matter.
The NIH, which describes itself as the “largest public funder of biomedical research in the world,” said it had good reason for approving such funds. Grant-making decisions were “supported by a substantial body of evidence” gathered during animal studies, the agency said in its statement.
The ongoing funding, however, has stoked a significant debate in the stem cell field over whether federal money is being squandered.

“Now that we know that adult stem cells do not regenerate the heart and that past work suggesting otherwise was false, why hasn’t this knowledge traversed its way through the medical and research systems, and why do such studies persist?” said Jeffery Molkentin, the director of molecular cardiovascular biology at Cincinnati Children’s Hospital.
Dr. Charles Murry, a longtime critic of Anversa who heads a lab at the University of Washington studying embryonic and adult stem cells, said the fabrication by Anversa’s lab has tarnished the whole discipline.
“This is a terrible black eye for our field,” he said. “But everyone is still pretending like it didn’t happen.”
Anversa’s case shows how a dramatic claim of scientific discovery can gain credibility and attract grants, private investment and backing even from world-class medical institutions despite evidence that the underlying research is flawed or faked. Even after core work is discredited, millions may continue to be spent on a questionable hypothesis, distorting the overall direction of scientific inquiry, specialists in research malfeasance say.
From the beginning, Anversa and his collaborators were able to drive the scientific narrative on the use of adult stem cells in heart regeneration, making their case in some of the most admired medical journals in the world. In the end, at least six journals issued a total of 19 retractions on papers produced by Anversa’s lab – often years after the original studies were published. They offered few details and limited context.
Meanwhile, an unknown number of heart patients were left in the dark, unaware of allegations of malfeasance as they decided whether to enroll in trials or stick with conventional treatment.
Though they eventually brought the Anversa scandal to the surface, Brigham and Harvard have yet to provide a full public accounting of what they know about the discredited research. Both declined to address questions about Anversa and his lab, saying research misconduct investigations are confidential.
Brigham and Harvard have never named the 31 papers with data they deemed fabricated or falsified nor identified the journals that received notices, and they declined to do so when asked by Reuters. However, the news organization was able to confirm the identity of 19 papers from Anversa’s lab that were eventually retracted.
The journals, which also included gold standard publications such as The Lancet and the New England Journal of Medicine, said they handled the matter in an appropriate way.
“This is a terrible black eye for our field. But everyone is still pretending like it didn’t happen.”
“Authors’ institutions are best placed to lead independent investigations into scientific misconduct,” The Lancet told Reuters.
After several unsuccessful efforts to reach Anversa, Reuters visited his New York City apartment building last month, where a reporter spoke to him from a lobby phone. Anversa, now 83, declined to comment, saying he “doesn’t want to bring it all up again.” The reporter also left a list of written questions that went unanswered.
In the past, Anversa has said that his adult stem cell research was valid and that a deputy was responsible for any alleged fabrications. He accused Brigham of attempting to hold on to his NIH grants.
After the Justice Department’s findings, Brigham agreed to pay NIH back $10 million , about a quarter of what Anversa’s lab received since 2008 for adult stem cell cardiac research. His lab closed in 2015.
The NIH said it takes “research misconduct very seriously,” but declined to comment on the Anversa case, saying it was a confidential matter.
The AHA, the largest non-profit funder of cardiovascular disease research in the United States, said it has spent $73.4 million of its own money for adult stem cell research since 2006, although it says it never funded Anversa directly.
Steven R. Houser, a cardiovascular scientist who was AHA president in 2016, said that the research was needed to test the potential of adult stem cells. “The cardiac stem cell hypothesis did not fall into disfavor because of the discovery of data fabrication by the Anversa lab,” he said. “It went away because of careful science.”
Advocates for continuing such research say the vast majority of adult stem cell studies on hearts has drawn no accusations of fabrication or bad faith, and that Anversa’s tainted work makes up a small fraction of papers in the field. Other small studies, they say, have shown real promise.
“The problem is there hasn’t been a big enough study on adult stem cells in hearts,” said Dr. Joshua Hare, the director of a stem cell institute at the University of Miami. “Why would we give up after so many years and investment?”
Hare did not do research with Anversa, nor were papers he authored retracted. He was, however, an editor of an Anversa paper that was withdrawn. Not counting collaborations with other researchers, he has received $29 million in NIH funding since 2000.
He acknowledged that he was deceived by Anversa. But “it wasn’t just me,” he said. “It was some of the most prominent people in the country who believed Piero Anversa.”
Anversa’s influence on his field was both extensive and enduring.
A Reuters review found that at least 5,000 people worldwide – including babies – have been included in privately and publicly funded adult stem cell studies on hearts in the past two decades.
“These kinds of cases are like scientific Ponzi schemes. Once you have that golden ticket, how do you stop cashing it in?”
The news organization also found that, over the same period, a network of adult stem cell researchers associated with Anversa served in top positions at scientific journals and on NIH grant committees, keeping the concept alive long after his lab’s fabrications came to light.
Anversa and other scientists also sought to profit from adult stem cell research in hearts, taking out patents and forging deals with private companies.
Political winds blew in their favor. Stem cells, basic cells that replace or repair diseased parts of the body, come in two major types: those found in embryos and those found in adults. Embryonic stem cells are far more versatile, with the ability to morph into all kinds of specialized cells. But their use, which involves destroying embryos, outrages abortion opponents. In 2001, the United States banned government funding for most embryonic stem cell research.
Adult stem cells can regenerate some parts of the body such as bone marrow to treat diseases like leukemia, but these cells are much more limited in their ability to reproduce and regenerate tissue.
Some scholars say that before more money from the NIH’s tight budget is spent on adult stem cell treatment for cardiac patients, the journals and institutions involved in the Anversa fabrication scandal should offer a fuller accounting of their role and find better ways to spot fabulists.
“These kinds of cases are like scientific Ponzi schemes,” said Marc Edwards, a professor at Virginia Tech who studies academic misconduct and fabrication. “Once you have that golden ticket, how do you stop cashing it in?”
An immediate buzz
For decades, most scientists believed that the heart, unlike skin or muscle, could not repair itself.
In 2001, Anversa upended that assumption.
In a paper published in the influential scientific journal Nature, Anversa and his co-authors concluded that a type of adult stem cell derived from bone marrow, known as c-kit positive stem cells, regenerated damaged heart tissue in mice.
The finding created immediate buzz, although the research was a long way from being validated in people. The paper was never retracted.
Five months after publication of the Nature study, under pressure from abortion opponents, U.S. President George W. Bush restricted most federal funding for embryonic stem cell research, and declared adult stem cells to be a “promising” alternative . The AHA, which had never funded embryonic stem cell research, formally banned it and quickly embraced Anversa’s concept. In 2003, it handed the doctor a “distinguished scientist” award .
In his 60s at the time, Anversa, who trained in his native Italy, was a professor at New York Medical College in the hamlet of Valhalla. Few scientists publicly questioned his sudden acclaim – or that of his co-authors. He joined forces at the college with Bernardo Nadal-Ginard , a former
chairman of Boston Children’s Hospital’s cardiology department, who had been declared by a U.S. criminal court judge to be “a common and notorious thief.”
Nadal-Ginard was released from jail in the late 1990s after serving nine months for misappropriating funds at Boston Children’s Heart Foundation. He was ordered to repay nearly $6.6 million to the charity. While still under court supervision in 1999, he began working at New York Medical College with Anversa, according to court records .
Nadal-Ginard became a regular co-author with Anversa, including on the landmark 2001 Nature paper . He also co-authored two New England Journal papers that were flagged as problematic by the Brigham-Harvard investigation. The journal said in a statement that it had posted “expressions of concern” – less serious than retractions – about the papers but did not withdraw them because the other co-authors were confident in the results.

“All stood behind the data,” said the journal, which did retract a 2011 paper of Anversa’s in which Nadal-Ginard played no role .
New York Medical College confirmed Nadal-Ginard left in 2005. It said in a statement that it couldn’t comment on the fabrication because of confidentiality rules and a change in the college’s leadership in 2011. The current officials “have never met nor ever had any communication with Dr. Anversa,” the college said.
Nadal-Ginard declined to comment.
Two other Anversa deputies, Jan Kajstura and Annarosa Leri, also began churning out adult stem cell papers. Leri declined to comment through her lawyer. Kajstura, the deputy whom Anversa had blamed for any potential fabrication, also declined to comment.
Other researchers, including people unaffiliated with Anversa, dived in after the Italian scientist’s landmark finding. Later in 2001, German researcher Bodo-Eckehard Strauer became the first scientist in the world to inject a human heart with adult stem cells. Strauer claimed after clinical trials that the patients’ heart scarring had improved by one-third.
The approach by Strauer and his colleagues attracted attention – even from the Vatican – because it side-stepped the abortion issue and offered new hope to heart patients. The United States spends more than $360 billion annually to treat cardiovascular disease, but conventional medicines can only modestly improve the quality of life for those with severe cases.
“Suddenly (Anversa) had celebrity status, and it became easier after that for him to get papers published and funding,” said Ferric C. Fang, a University of Washington microbiologist who has studied scientific journal retractions . “Because who’s going to want to turn down this guy who could be saving the world from heart disease?”
‘Unbelievably charming’
The publicity, including glowing headlines, brought financial investment.
According to one analysis published by the UK’s national academy of sciences , the worldwide capital value of publicly traded companies in the regenerative medicine field was $4.7 billion in 2007, more than 15 times higher than four years earlier. By then, firms focusing on adult stem cells – not just in heart patients – made up more than 60% of the market.
As NIH grants poured in, Anversa filed three dozen adult stem cell patents, including some with Brigham and New York Medical College, and one with the federal government.
Anversa left the college to head his own lab at Brigham in 2007. He became the most prominent among a growing group of researchers known for their fierce advocacy of adult stem cell treatments in hearts.
In a small and sometimes insular field, these researchers were often in a position to support one another, either as journal editors or members of NIH grant-making panels. Anversa served on an NIH advisory board , as well as an NIH grant review panel.
He was “unbelievably charming” and persuasive, said University of Washington’s Dr. Robb MacLellan, who served with Anversa on the same grant committee but described himself as skeptical of Anversa’s work because no one could replicate his results. Anversa, he said, was able to “package everything up in a true-believer sort of way and sell it.”
One Anversa research collaborator, Dr. Roberto Bolli of the University of Louisville , served on six NIH grant review panels that funded stem cell research on hearts.
Mark Sussman, a biologist at San Diego State University, served on eight such NIH grant committees while publicly talking up Anversa as a pioneer in the “concept of the heart as a regenerative organ.”
Between 2001 and 2021, the three scientists became among the top 20 principal researchers to collect NIH funding aimed at studying adult stem cell treatment for hearts.
As a solo investigator, Anversa received $45 million in grants. Also solo, Bolli was allotted $59 million and Sussman $35 million. All told, the three accounted for more than a third of the $387 million total allocated to the top 20 investigators on the subject during that period.
NIH committee members are not permitted to weigh in on their own lab’s grants or those of their collaborators. NIH officials declined to respond to questions about individual grant decisions, or the timing of individual committee memberships.
Bolli declined to discuss NIH committee memberships. However, in response to questions about Anversa, he said he had no knowledge of the doctor’s fabrications while working with him.
“I was a victim of that fraud,” Bolli said.
“Needless to say, the fabrication in the Anversa laboratory has been a tragedy and has caused immense damage, not only to the field of stem cells and heart disease, but to science in general,” he added.
Sussman said that his collaboration with Anversa was “limited,” and then cut short a phone conversation with a reporter. He and San Diego State did not respond to follow-up calls or emails.

Anversa and his collaborators also sat on editorial boards of the high-profile AHA journals that published adult stem cell research.
Bolli was editor-in-chief of Circulation Research between 2009 and 2019. And Joseph Loscalzo, also an Anversa collaborator and the chair of Brigham ’s Department of Medicine since 2005, was editor of Circulation between 2004 and 2016.
All told, Circulation Research and Circulation published hundreds of pieces about cardiac adult stem cell research, including more than 300 that cited Anversa’s work, a Reuters review found.
Fourteen of 56 articles from Anversa’s lab in those two journals alone were retracted as a result of the Brigham-Harvard probe, including one co-authored with Bolli and three with Loscalzo.
Through a Brigham spokesman, Loscalzo declined to comment.
In its statement to Reuters, the AHA said it is responsible for having papers carefully reviewed by peers, but the conclusions “are solely those of the study authors,” and the AHA “makes no representation or guarantee as to their accuracy or reliability.”
In the case of Anversa, it said, “the scientific process worked and identified the extent of the fraud, and remedies, including retraction, were duly implemented.”
‘No one likes to admit it’
The research giants – including Brigham, Harvard and the NIH – were slow to catch on to the fabrication from Anversa’s lab. Part of the explanation lies in the arcane nature of the field, one expert on research misconduct said.
“No one likes to admit it, but few people really understand this sort of highly specialized research except for a handful of scientists,” said Fang, the researcher who studies retractions. “Even the deans, department heads and journal editors can struggle to know if something is hype or reality. And if (researchers are) lying about data, it’s almost impossible to catch it.”
Harvard began to hear from skeptics of Anversa’s work in 2009, however, as the medical school considered him for a professorship.
In a letter that year to Harvard Medical School reviewed by Reuters, Murry, the stem cell researcher and longtime Anversa critic, offered a warning.

Murry acknowledged that the medical school would be gaining “a professor who brings in large amounts of funding, publishes volumes of influential work and brings a spotlight on your school and affiliated hospitals.”
But he cautioned that “Harvard will also lend its good name to this controversial work and the clinical trials that it generates.”
Dr. Jeffrey Flier, who became dean of Harvard Medical School in 2007, said that he and the hiring committee conferred for months. After hearing from more admirers than critics, Flier said, he recommended the appointment and Harvard’s provost approved it . Flier, however, said he asked Brigham’s leaders to keep a close eye on Anversa’s work.
“I was told he was doing great, with no problems,” Flier said.
Exaltation and suspicion
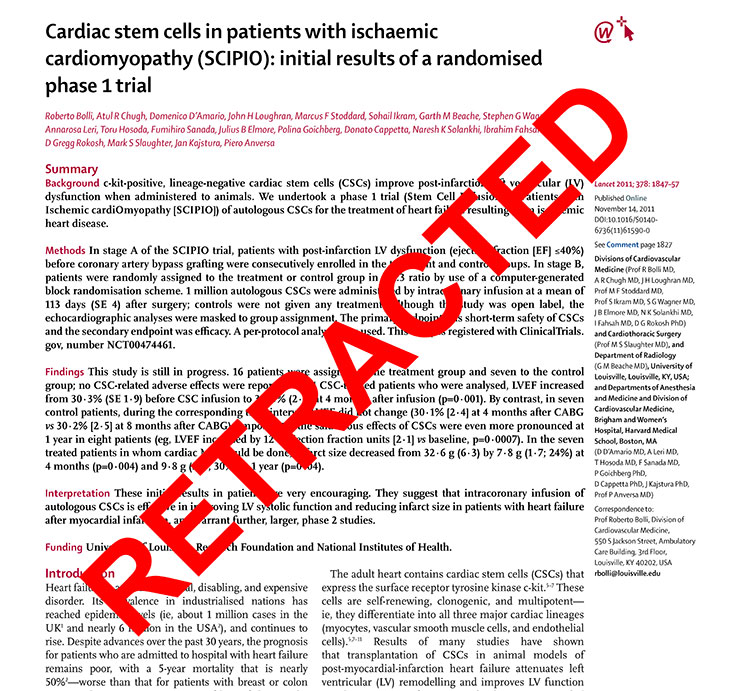
Anversa and others plowed ahead with their research. In 2011, a group that included Bolli, Anversa and Kajstura advanced to human trials with the so-called SCIPIO project – named after the illustrious ancient Roman general . The first stage involved injecting 16 patients’ hearts with c-kit positive stem cells.
At that November’s AHA conference, Anversa and Bolli presented early results, purportedly showing an increase in heart function and reduction in scar tissue. Bolli hailed the preliminary, or Phase 1, findings in his university’s press release as possibly the “biggest revolution in cardiovascular medicine in my lifetime.”
But by the summer of 2011, researchers inside Anversa’s lab had begun to share concerns about potential fabrication, according to five former Anversa lab members. “I came in with a very hopeful view of their research,” recalled Nathan Tucker, then a biologist in the lab. “Within two months, I had come to believe that a vast majority of what was going on was not what they said it was.”
Tucker and Ricciardi said they suspected that images of cells had been altered to support Anversa’s published assertions.

In many instances, while trying to isolate adult stem cells with regenerative properties from the heart tissue, they were unable to find the c-kit positive stem cells that formed the foundation of the lab’s work, Tucker said.
“Yet someone would do the same thing the next day and have a ton of them,” recalled Tucker.
Around the same time, said Tucker, lab workers – many of them inexperienced – told him how they were “recounting” or “reanalyzing” data to “do it right.” That fiddling, he said, was a potential sign of data manipulation.
In November 2012, eight researchers expressed their worries to Brigham officials, according to emails between the lab members and hospital officials that were reviewed by Reuters.
Days later, coincidentally, Harvard Medical School received a letter from Lawrence Livermore National Laboratory in California, calling into question a paper on the regeneration concept by Anversa and Loscalzo, which was edited by Hare . The letter said the work had misrepresented data gathered by one of Livermore’s researchers.
The researcher, Bruce Buchholz, confirmed to Reuters that the letter was sent on his behalf, saying it detailed how data he provided to Anversa’s lab had been altered, without his knowledge, to include measurements he never made. The study was later retracted by the AHA’s Circulation.
Advocates of adult stem cell research, including the Vatican, continued to rally behind the field and its scientists. Beginning in 2011, the Vatican highlighted adult stem cells in its scientific conferences, citing Bolli’s research in its materials.
Fabrication spreads
Evidence accumulated of flaws and fabrication by other researchers.
In 2013, a group of researchers published a critique of work by Strauer, the German scientist unaffiliated with Anversa who oversaw the first human trials. The group reviewed 48 papers from his lab and reported finding 200 serious “discrepancies,” including exaggerated or missing data.
A year later, the University of Dusseldorf found evidence of scientific misconduct against Strauer, who by then had retired. A university spokesman told Reuters the allegations involved violations of rules governing trials and publications but said he could not comment further, citing confidentiality restrictions. The trials stopped with the departure of Strauer, who could not be reached for comment.
The journal Nature also retracted a paper by another high-profile Brigham researcher – unassociated with Anversa – that found adult stem cells had regenerative properties in various human tissues. That resulted in a rare apology from the journal, saying that research institutions and journals need to “ensure that the money entrusted by governments is not squandered, and that citizens’ trust in science is not betrayed.”
Meanwhile, the Federal Bureau of Investigation began to examine the Brigham whistleblowers’ allegations, according to emails between them and the hospital that were reviewed by Reuters.
Brigham and Harvard widened their own investigation as more scientific papers were thrown into question. Flier, who said he regularly asked about the status of the inquiry, checked in again before stepping down as dean.
“I was told they hoped it would be done,” he recalled.
When Flier left his post in July 2016, it still wasn’t completed.
‘Far-reaching consequences’
In October 2018, nearly six years after beginning their inquiry, Brigham and Harvard briefly announced its completion. They offered no details on what research was falsified nor where it appeared but said they had alerted the journals involved.
“A bedrock principle of science is that all publications are supported by rigorous research practices,” the Brigham-Harvard statement said. Without them, “there are far-reaching consequences for the scientific enterprise.”
None of the 19 retractions provides context on what was wrong or how the malfeasance occurred. In addition to the retractions, three journals issued “expressions of concern” for four papers because of suspected data or image manipulation – advisories less severe than retractions.
The lengthy investigation and the delays in retractions meant some patients did not get wind of the ongoing Brigham-Harvard investigation even as they were being enrolled in new trials.
For instance, The Lancet issued “an expression of concern” about the SCIPIO trial in 2014, based on the ongoing Brigham-Harvard probe. Despite The Lancet’s concerns, Bolli and the University of Louisville touted the success of SCIPIO in a university publication in 2016, portraying it as “a landmark” trial that set the stage for a new and larger study.
The approximately 125 patients enrolled nationwide in the second trial, known as “CONCERT-HF,” were not informed of SCIPIO’s problems until December 2018, after the Brigham-Harvard inquiry ended, the NIH confirmed. By then, a CONCERT-HF patient had died of a heart perforation during 2016 trial preparations.
When the Lancet eventually retracted the SCIPIO paper in 2019, the journal said the Brigham-Harvard inquiry results “persuade us that the laboratory work undertaken by Piero Anversa and colleagues at Harvard cannot be held to be reliable.”
The Lancet, however, found that Bolli’s lab relied on the results in “good faith.”
In a statement to Reuters, Bolli was as effusive about CONCERT-HF as he once was of SCIPIO, calling it “arguably the most rigorous cell therapy trial ever conducted in heart disease.”
As Anversa’s career fizzled, Bolli, who co-authored three studies with him that were ultimately retracted, remained the editor of Circulation Research until 2019.
He departed not because of the fabrication scandal but due to an unrelated controversy over an antigay email he sent to a ballet company . The AHA said it “relieved” him of his duties as a result of language “alleged to be hate speech.” Bolli, who did not respond to questions about the incident, said at the time that his views didn’t affect his treatment of patients.
“It’s heartbreaking,” said researcher Ricciardi, who has since received a lung transplant and now lives in Italy. “So many sick people were given false hope for so many years.”
Those involved in adult stem cell research in hearts maintain the field has moved on from the Anversa scandal. A promising new method reprograms adult stem cells into an embryo-like state.
Bolli and several former Anversa collaborators continue to receive millions of dollars in NIH grants. Of the $59 million Bolli collected in the past two decades as a solo investigator on adult stem cell research in hearts, $11.4 million was allocated between 2018 and 2021.
More than $1.8 million in NIH funding has gone to Hare, the University of Miami researcher, and others for research aimed at healing a deadly cardiac disease in infants by injecting adult stem cells into their hearts. Hare’s company is trying to get U.S. approval for the therapy.
The NIH said notifying participants’ parents of prior fabrication in the field was “not relevant” because the trial did not rely on Anversa’s work.
It’s not fixed yet
Almost four years after the Brigham-Harvard investigation ended, it remains unclear which Anversa papers were examined for fabrication.
Nature confirmed that Brigham and Harvard never contacted it about Anversa’s landmark 2001 regeneration paper, which included an NIH staff scientist as co-author. Spokesman Michael Stacey declined to say whether the journal scrutinized the paper on its own, only that it takes any concerns seriously and looks into them “carefully.”
Brigham and Harvard were required to share a copy of their 2018 findings with the U.S. Office of Research Integrity (ORI) , tasked with investigating scientific misconduct.
Through a spokesperson, the agency declined to respond to questions, including whether it investigated the matter.
Flier said ORI’s silence on what he called Harvard’s “biggest research scandal in recent history” suggests that the federal “system for responding to such investigations is broken.”
Ricciardi, the molecular biologist once so excited to join Anversa’s team at Brigham, says he is appalled that so little has changed in the decade since he and his labmates blew the whistle.
Anversa’s fabrication had felt like a personal blow. Ricciardi, who has the life-threatening lung disorder cystic fibrosis, said he originally was inspired to join the lab because of an Anversa paper citing evidence that lungs, as well as hearts, might be healed using adult stem cells.
Seven years later, the paper was retracted by the New England Journal , which said images had been manipulated.
“It’s heartbreaking,” said Ricciardi, who has since received a lung transplant and now lives in Italy. “So many sick people were given false hope for so many years.”